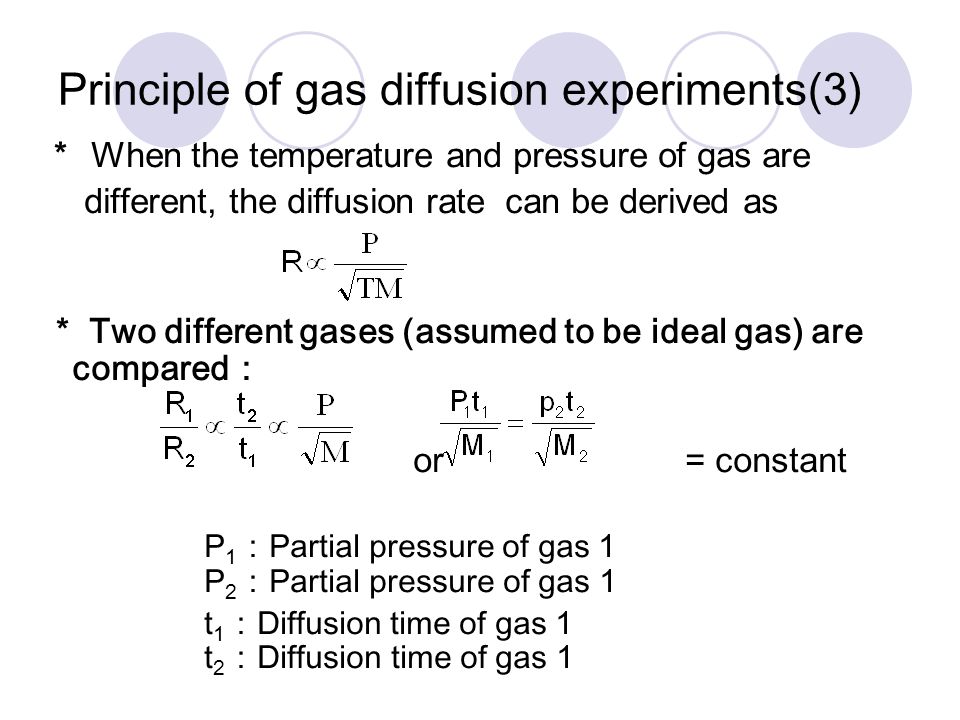
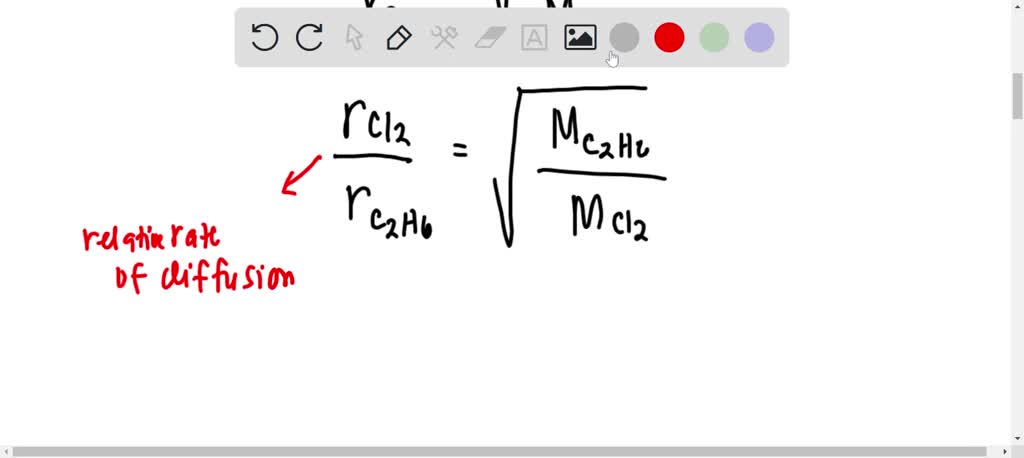
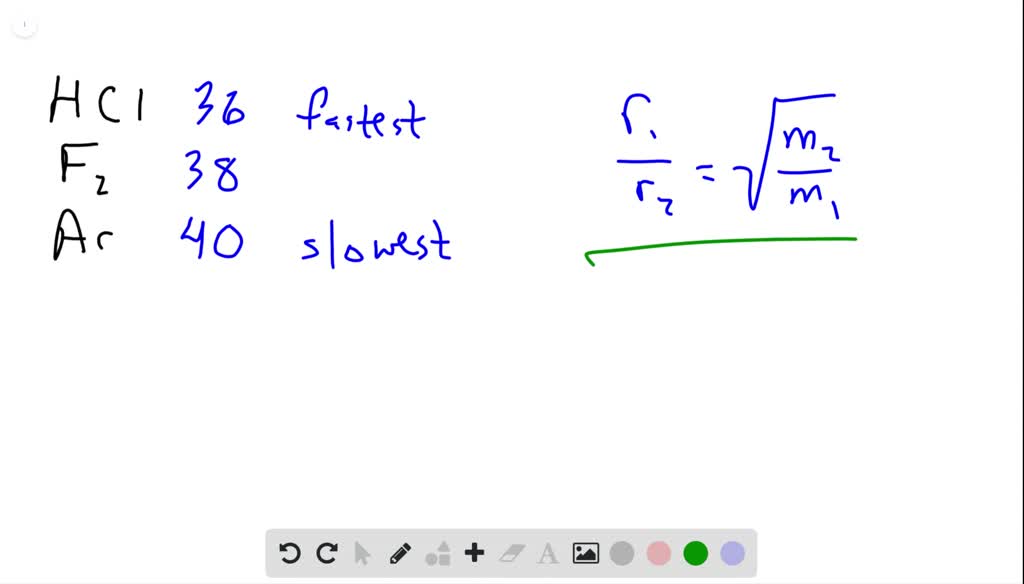
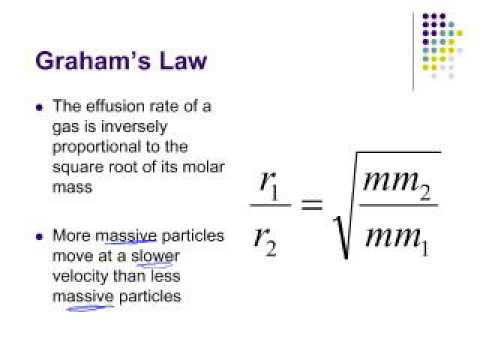
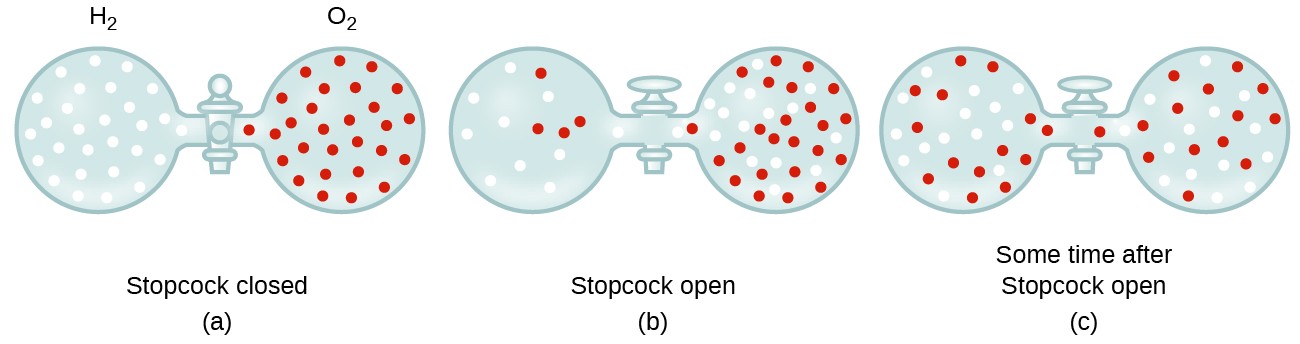
Rate of diffusion of a saturated hydrocarbon is about 1/6 th of that of hydrogen under similar conditions of temperature and pressure. What is the molecular formula of that hydrocarbon?
34. if the ratio of Rates of diffusion of two gases X and Y is 9 : 1 the ratio of their densities is

Rates of diffusion of two gases A and B are in the ratio 2:1. If the molecular mass of gas A is 16g. Find the...

The rates of diffusion of gases A and B of molecular weight 36 and 64 are in the ratioa)9:16b)4:3c)3:4d)16:9Correct answer is option 'B'. Can you explain this answer? - EduRev JEE Question









![ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz ANSWERED] SolveLancer Test The rate of diffusion of two gases X Y are - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210625230951982656-1884508.jpg)